From back pain to cancer diagnosis
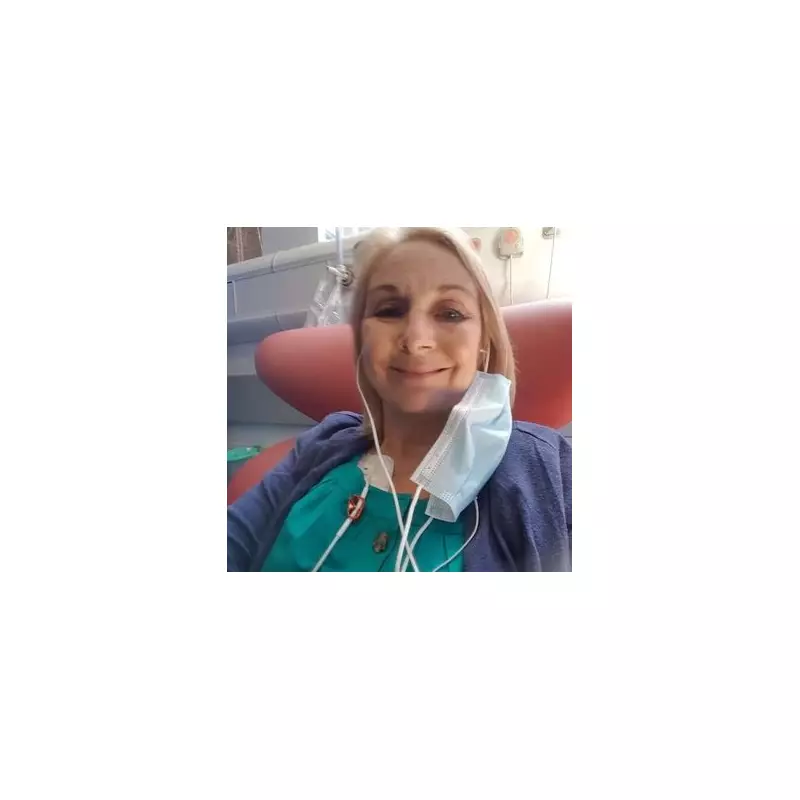
Sue Harley, a 63-year-old grandmother from Kings Heath, initially believed her severe back pain was simply part of ageing. What began in 2015 as discomfort eventually became so debilitating that she struggled to walk even ten steps on some days.
Her symptoms were repeatedly misdiagnosed over two years, with doctors initially suggesting osteoarthritis before wrongly identifying her condition as metastatic breast cancer around Christmas 2016. The truth emerged in January 2017 when specialists confirmed she had myeloma, an incurable blood cancer affecting more than 33,000 people in the UK.
The long road to effective treatment
Over the next six years, Sue underwent five consecutive treatments, including travelling to India to access lenalidomide when it wasn't available on the NHS. None of these approaches provided lasting remission.
By 2023, with her cancer returning for the fifth time, Sue had exhausted conventional treatment options. That's when she gained access to talquetamab through a compassionate use programme, a next-generation bispecific antibody treatment not yet approved for widespread NHS use.
The drug works by binding to both myeloma cells and T-cells, helping the immune system recognise and destroy cancer cells. Clinical trials showed nearly 60% of patients experienced a very good partial response or better.
Life transformed and NHS victory
Two years after starting talquetamab in September 2023, Sue remains in remission - her longest period without active cancer since diagnosis. This groundbreaking treatment has enabled her to run two half-marathons, maintain an active gym routine, participate in book clubs, volunteer weekly, and most importantly, enjoy precious time with her five-year-old granddaughter and six-month-old grandson.
"When I was diagnosed, I didn't know if I would live to see grandchildren, let alone live to see them grow up," Sue reflected.
She joined forces with charity Myeloma UK to campaign for NHS approval of talquetamab, resulting in a significant victory when the National Institute for Health and Care Excellence approved the treatment for use in England and Wales from November 17.
Shelagh McKinlay, Myeloma UK's Director of Research and Advocacy, emphasised the importance of this development: "Talquetamab offers more flexibility and leads to a better quality of life. Its unique mechanism of action means it can fight myeloma in a completely different way."
The approval means up to 800 patients annually in England and Wales can benefit from this life-extending treatment, specifically designed for people whose cancer has returned at least three times after previous therapies.
For Sue, the impact extends beyond statistics. "This is my sixth line of treatment and there's that hope this is the thing that's going to work for me forever," she said. "You can't underestimate how important having hope is."